-
-
GLP-1 Side Chains
 显示更多
显示更多 -
Peptide Intermediates

-
Fmoc-His-Aib-OH
-
Fmoc-Ile-Aib-OH
-
Fmoc-Tyr(tBu)-Aib-OH
-
Fmoc-Glu(OtBu)-Aib-OH
-
Fmoc-His(Fmoc)-Aib-OH
-
Fmoc-His(Fmoc)-Aib-OSu
显示更多 -
-
Tirzepatide Fragments

-
CAS:2682040-93-1
-
CAS:3034670-52-2
-
CAS:2656383-23-0
-
CAS:2461524-68-3
-
CAS:2656383-24-1
-
CAS:2656383-25-2
显示更多 -
-
API & Excipients
 显示更多
显示更多 -
Cosmetic Peptides
-
Oligopeptide-1
-
NonaPeptide-1
-
Copper Tripeptide-1
-
Acetyl Hexapeptide-8
-
Palmitoyl Tripeptide-1
-
Decarboxy Carnosine HCl
显示更多 -
-
Active Ingredients
 显示更多
显示更多
-
Application and Value of AEEA-AEEA (CAS1143516-05-5): An Indispensable Intermediate for Synthesizing GLP-1 Agonists
Publish Time:
2025-08-20
With the popularity of semaglutide in the weight loss field, multiple GLP-1 receptor (GLP-1R) agonists have been successively developed, and many domestic pharmaceutical companies have also devoted themselves to the research, development, and production of this type of drug. However, the core challenge in the development of these drugs lies in the naturally short half-life of GLP-1 — the half-life of GLP-1 secreted by the human body is only 1 to 2 minutes, and it is easily degraded by dipeptidyl peptidase 4 (DPP-4) after entering the bloodstream, thereby losing its insulin secretion-promoting activity. Therefore, developing long-acting formulations (including injectable and oral forms) to extend the half-life has become a key direction to promote technological breakthroughs and product iteration of GLP-1R agonists.
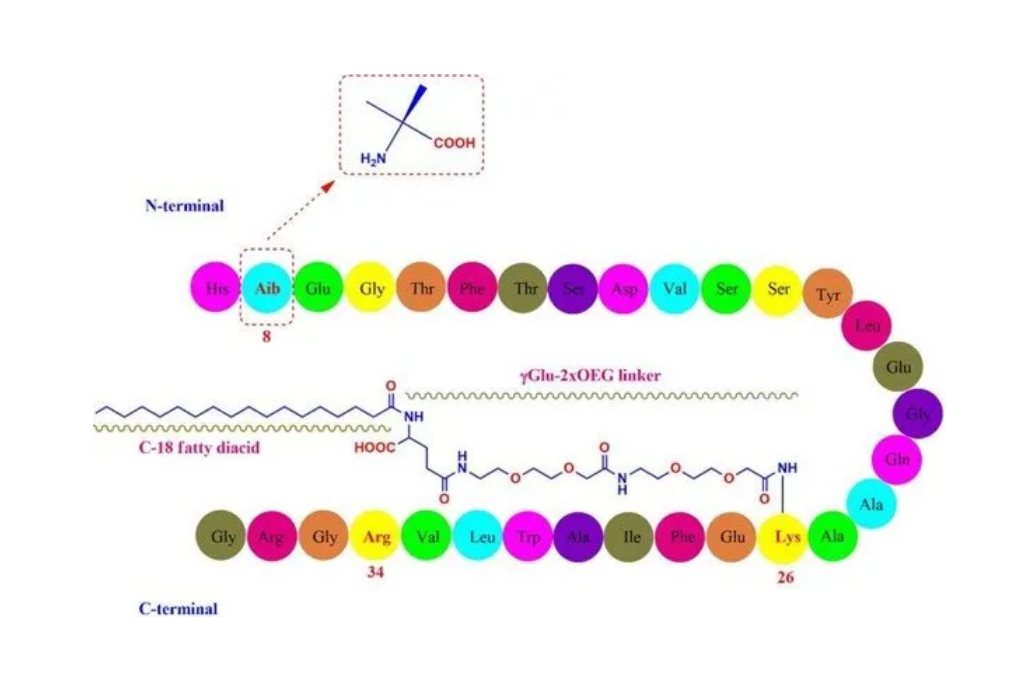
Core long-acting strategies for GLP-1 long-acting formulations
Currently, the long-acting technology pathways for GLP-1 long-acting formulations are mainly divided into three categories:
1. Amino acid site modification to resist DPP-4 degradation
By altering the structure of the 8th amino acid at the N-terminus of the peptide, the cleavage action of DPP-4 can be prevented. Studies have shown that DPP-4 prefers to cleave peptides with proline (Pro) or alanine (Ala) at the 8th position of the N-terminus, while peptides with modified 8th amino acids (such as the introduction of Aib) exhibit significant resistance to DPP-4 degradation. For example, the half-life of natural GLP-1 (7-36)-NH₂ is less than 2 minutes, whereas the half-life of modified analogs can be greatly extended.
2. Introduction of macromolecular modifications to slow renal clearance
By introducing fatty acid chains to promote binding with albumin, thereby reducing the rate of renal clearance; or by fusing GLP-1 with macromolecules such as albumin, or covalently linking with polyethylene glycol (PEG), increasing molecular size to slow renal elimination (as shown in Figure 2). For example, connecting a C18 fatty diacid side chain to a specific peptide site via a spacer can enhance the molecule's binding affinity to albumin, while precise modification sites can avoid incorrect fatty acid binding positions, further optimizing the long-acting effect.
3. Application of sustained-release technology to maintain stable blood drug concentration
By controlling the drug release rate through subcutaneous sustained-release technology (such as microsphere formulation technology), the stability of drug concentration in the blood is maintained, thereby prolonging the duration of action.
Key intermediate AEEA-AEEA: the core connecting point between long-acting strategies and drug synthesis
Among the marketed GLP-1R agonist drugs, when fatty acid chains are introduced through amino acids to achieve long-acting effects, PEG-type linkers are often used, with H-AEEA, AEEA-AEEA, and AEEA-AEEA-AEEA being common types. Specifically, liraglutide uses H-AEEA as the linker; semaglutide and tirzepatide use AEEA-AEEA as the core linker; some generic drugs under development may use AEEA-AEEA-AEEA as a modified linker to optimize the structure.
Basic information of AEEA-AEEA
The chemical name of AEEA-AEEA is 17-amino-10-oxo-3,6,12,15-tetraoxa-9-azaoctadecanoic acid, with the structural formula as follows:
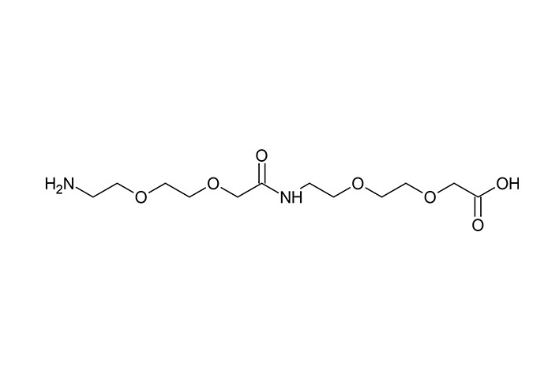
Its core information is shown in the table below:
| Item | Details |
| Appearance | White or off-white powder |
| CAS No. | 1143516-05-5 |
| Molecular formula | C12H24N2O7 |
| Molecular weight | 308.33 |
| AEEA | ≤0.1% |
| AEEA-AEEA-AEEA | ≤0.1% |
| Purity | ≥99% |
| Manufacturer | Guangzhou Congen Pharmatec Co.,Ltd. |
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
These cookies are necessary for basic functions such as payment. Standard cookies cannot be turned off and do not store any of your information.
These cookies collect information, such as how many people are using our site or which pages are popular, to help us improve the customer experience. Turning these cookies off will mean we can't collect information to improve your experience.
These cookies enable the website to provide enhanced functionality and personalization. They may be set by us or by third-party providers whose services we have added to our pages. If you do not allow these cookies, some or all of these services may not function properly.
These cookies help us understand what you are interested in so that we can show you relevant advertising on other websites. Turning these cookies off will mean we are unable to show you any personalized advertising.
Sorry,当前栏目暂无内容!
您可以查看其他栏目或返回 首页
Sorry,The current column has no content!
You can view other columns or return Home




