-
-
GLP-1 Side Chains
 显示更多
显示更多 -
Peptide Intermediates

-
Fmoc-His-Aib-OH
-
Fmoc-Ile-Aib-OH
-
Fmoc-Tyr(tBu)-Aib-OH
-
Fmoc-Glu(OtBu)-Aib-OH
-
Fmoc-His(Fmoc)-Aib-OH
-
Fmoc-His(Fmoc)-Aib-OSu
显示更多 -
-
Tirzepatide Fragments

-
CAS:2682040-93-1
-
CAS:3034670-52-2
-
CAS:2656383-23-0
-
CAS:2461524-68-3
-
CAS:2656383-24-1
-
CAS:2656383-25-2
显示更多 -
-
API & Excipients
 显示更多
显示更多 -
Cosmetic Peptides
-
Oligopeptide-1
-
NonaPeptide-1
-
Copper Tripeptide-1
-
Acetyl Hexapeptide-8
-
Palmitoyl Tripeptide-1
-
Decarboxy Carnosine HCl
显示更多 -
-
Active Ingredients
 显示更多
显示更多
-
AEEA-AEEA Pharmaceutical Intermediates Professional Manufacturer |CongenPharma
Publish Time:
2025-08-19
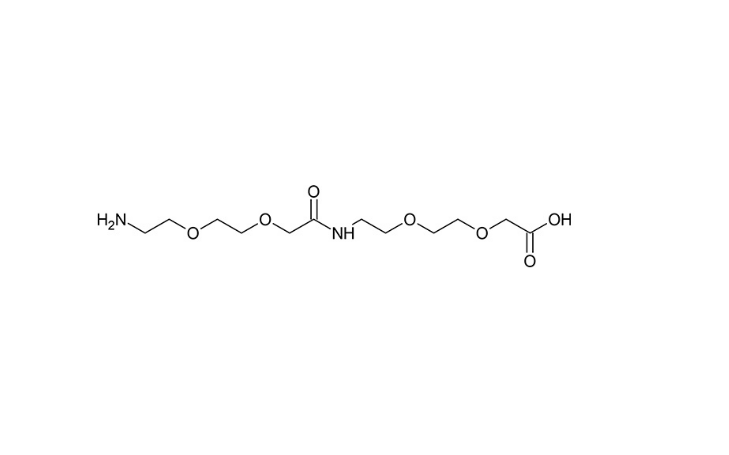
AEEA-AEEA structural formula
As a professional manufacturer of pharmaceutical intermediate AEEA-AEEA,CongenPharma has redesigned the process route of AEEA, and adopted the amino-carboxyl synergistic protection-regeneration route, using diethanolamine and acyl chloride as raw materials, and obtained AEEA and diAEEA through three steps of amidation, condensation, and hydrolysis. (CAS No.: 1143516-05-5) mixture, and then AEEA and diAEEA pure products are obtained through sieving and crystallization respectively, and a related patent is submitted, application number: 202510057484.1.
The process reduces the use of protecting and deprotecting reagents, has high atom economy, and the raw materials are easy to obtain. The production cost of AEEA is 50% of the current commonly used process, and AEEA-AEEA (CAS No.: 1143516-05-5) The production cost is 30% of the existing production process, which has significant AEEA-AEEA price advantages.
Based on the process, Tongjun Pharmaceutical Intermediate AEEA-AEEA (CAS No.: 1143516-05-5) has fewer related impurities, and the product quality far exceeds that of currently marketed products, with a purity of ≥99%, AEEA, 3AEEA≤0.1% (mole percentage).

CongenPharma peptide intermediate manufacturer |AEEA-AEEA Product picture
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
These cookies are necessary for basic functions such as payment. Standard cookies cannot be turned off and do not store any of your information.
These cookies collect information, such as how many people are using our site or which pages are popular, to help us improve the customer experience. Turning these cookies off will mean we can't collect information to improve your experience.
These cookies enable the website to provide enhanced functionality and personalization. They may be set by us or by third-party providers whose services we have added to our pages. If you do not allow these cookies, some or all of these services may not function properly.
These cookies help us understand what you are interested in so that we can show you relevant advertising on other websites. Turning these cookies off will mean we are unable to show you any personalized advertising.
Sorry,当前栏目暂无内容!
您可以查看其他栏目或返回 首页
Sorry,The current column has no content!
You can view other columns or return Home




