-
-
GLP-1 Side Chains
 显示更多
显示更多 -
Peptide Intermediates

-
Fmoc-His-Aib-OH
-
Fmoc-Ile-Aib-OH
-
Fmoc-Tyr(tBu)-Aib-OH
-
Fmoc-Glu(OtBu)-Aib-OH
-
Fmoc-His(Fmoc)-Aib-OH
-
Fmoc-His(Fmoc)-Aib-OSu
显示更多 -
-
Tirzepatide Fragments

-
CAS:2682040-93-1
-
CAS:3034670-52-2
-
CAS:1262308-37-1
-
CAS:2461524-68-3
-
CAS:2656383-24-1
-
CAS:2656383-25-2
显示更多 -
-
API & Excipients
 显示更多
显示更多 -
Cosmetic Peptides
-
Oligopeptide-1
-
NonaPeptide-1
-
Copper Tripeptide-1
-
Acetyl Hexapeptide-8
-
Palmitoyl Tripeptide-1
-
Decarboxy Carnosine HCl
显示更多 -
-
Active Ingredients
 显示更多
显示更多
-
AEEA-AEEA: A Vital Intermediate for GLP-1 Agonist Synthesis
Publish Time:
2025-08-01
With the popularity of semaglutide in the field of weight loss, a variety of GLP-1R agonists have been developed one after another, and many pharmaceutical companies have also joined the research and development and production of GLP-1R agonists. The biggest challenge in its research and development comes from its too short half-life. The half-life of GLP-1 secreted by the human body is only 1~2 minutes. After secretion into the blood circulation, it is easily degraded by dipeptidyl peptidase 4 (DPP-4) and loses its activity in promoting insulin secretion. Therefore, constantly updating the long-acting mechanism (injection, oral) to increase the half-life has become a technical difficulty that needs to be overcome in the research and development of GLP-1R agonists and an internal driving force for promoting product updates and iterations.
The strategies for the long-acting of GLP-1 long-acting preparations are mainly divided into three categories:
1) By changing the amino acid at position 8, the cleavage of DPP-IV is prevented, as shown in Figure 1 below;
2) By introducing fatty acid chains, fatty acids are bound to albumin, thereby slowing down renal clearance, or by fusing GLP-1 with macromolecules such as albumin, or by covalently binding with PEG, thereby slowing down renal elimination, as shown in Figure 2 below;
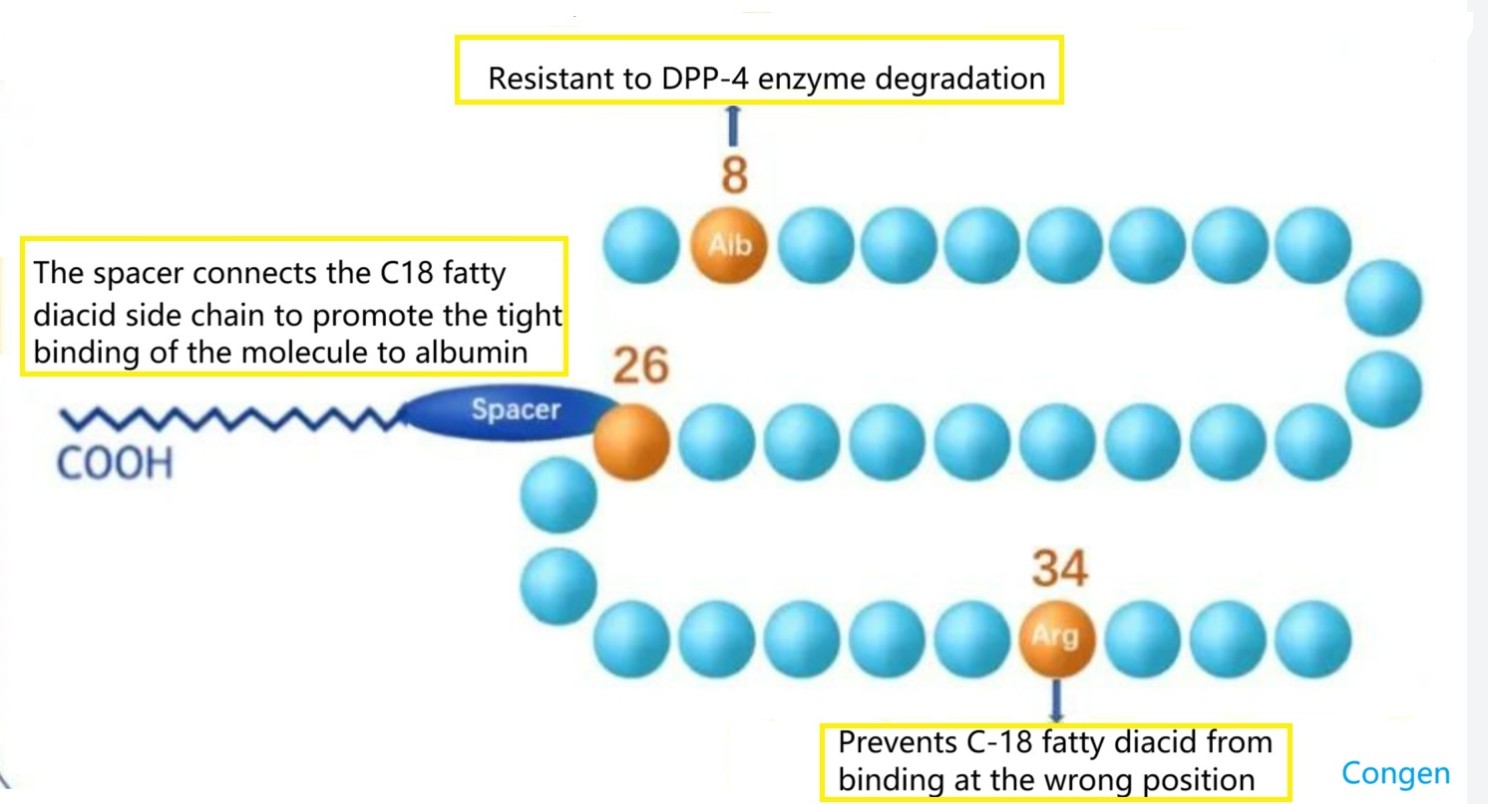
3) Maintaining blood drug concentration through subcutaneous sustained release, such as microsphere technology.
By analyzing the GLP-1R agonist drugs that have been marketed, PEG-type linking arms are usually used when introducing fatty acid chains on xenon-based acids. For example, H-AFFA AEFA-AFFA AFEA-AFEAAEEA. Among them, retaglutide uses H-AEEA as a linker; semaglutide and telpotide use AEEA-AEEA as a linker; other generic drugs in the research and development stage may use AEEA-AEEA-AEEA as a modified linker.
AEEA-AEEA is not only an important intermediate of semaglutide, but also used to synthesize the fatty acid side chains of peptide drugs such as semaglutide and Tirzepatide. It can also be used as a non-cleavable ADC linker for the synthesis of antibody-drug conjugates (ADCs)1]; it is also an alkyl chain-based PROTAC linker that can be used in the synthesis of PROTACs[2].
AEEA-AEEA is an important intermediate of GLP-1 agonists, and its usage has increased dramatically. Congen Pharma has conducted detailed quality research on this product and developed a unique scale-up process suitable for large-scale production to meet customer needs. For more product information, please contact marketing@congenpharm.com
Table 1. AEEA-AEEA basic information
Prev:
Relevant Information
undefined
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
These cookies are necessary for basic functions such as payment. Standard cookies cannot be turned off and do not store any of your information.
These cookies collect information, such as how many people are using our site or which pages are popular, to help us improve the customer experience. Turning these cookies off will mean we can't collect information to improve your experience.
These cookies enable the website to provide enhanced functionality and personalization. They may be set by us or by third-party providers whose services we have added to our pages. If you do not allow these cookies, some or all of these services may not function properly.
These cookies help us understand what you are interested in so that we can show you relevant advertising on other websites. Turning these cookies off will mean we are unable to show you any personalized advertising.
Sorry,当前栏目暂无内容!
您可以查看其他栏目或返回 首页
Sorry,The current column has no content!
You can view other columns or return Home




