-
-
GLP-1 Side Chains
 显示更多
显示更多 -
Peptide Intermediates

-
Fmoc-His-Aib-OH
-
Fmoc-Ile-Aib-OH
-
Fmoc-Tyr(tBu)-Aib-OH
-
Fmoc-Glu(OtBu)-Aib-OH
-
Fmoc-His(Fmoc)-Aib-OH
-
Fmoc-His(Fmoc)-Aib-OSu
显示更多 -
-
Tirzepatide Fragments

-
CAS:2682040-93-1
-
CAS:3034670-52-2
-
CAS:2656383-23-0
-
CAS:2461524-68-3
-
CAS:2656383-24-1
-
CAS:2656383-25-2
显示更多 -
-
API & Excipients
 显示更多
显示更多 -
Cosmetic Peptides
-
Oligopeptide-1
-
NonaPeptide-1
-
Copper Tripeptide-1
-
Acetyl Hexapeptide-8
-
Palmitoyl Tripeptide-1
-
Decarboxy Carnosine HCl
显示更多 -
-
Active Ingredients
 显示更多
显示更多
-
GLP-1 weight loss drug market dynamics: changes in leading drugs and policy benefits
Publish Time:
2025-07-06
Currently, GLP-1 weight-loss drugs are experiencing explosive growth in global and Chinese markets, and the industry is entering a golden period of development. What changes have occurred recently in this multi-billion dollar market competition? Let's take a look!
Shifting of Top-Selling Drugs: Semaglutide Surpasses Keytruda
Novo Nordisk's financial report shows that in the first quarter of 2025, semaglutide achieved sales revenue of US$7.864 billion, while Merck's Keytruda achieved revenue of US$7.205 billion. Semaglutide's single-quarter sales exceeded Keytruda for the first time, becoming the new "top-selling drug".
Eli Lilly's tirzepatide, which was launched slightly later, also achieved sales of US$6.15 billion in the first quarter of 2025.
Policy Benefits: WHO to Include GLP-1 in Obesity Treatment Guidelines
The World Health Organization (WHO) website published a notice in August 2024 soliciting opinions on guidelines for the use and indications of GLP-1 RA in adult obese patients. According to a recent report by People's Finance, guidelines for the treatment of adult obesity with GLP-1 RA and GLP-1 RA/GIP dual receptor agonists are currently being developed under the Guideline Review Committee procedure, and the final plan is expected to be announced in August or September 2025.
Market analysis indicates that GLP-1 drugs have growth potential exceeding market expectations, and the implementation of the WHO obesity guidelines is expected to be a new catalyst for further increasing GLP-1 drug sales.
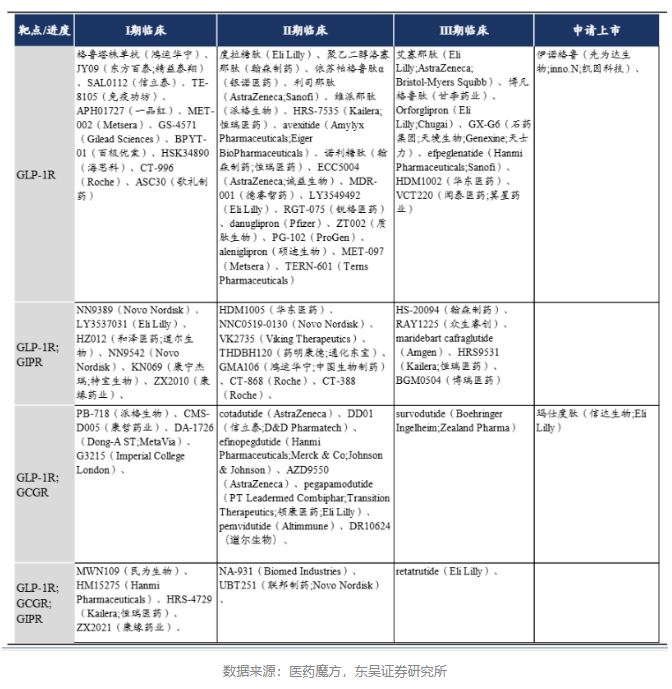
Competitive Landscape of GLP-1 Weight-Loss Drugs Under Development
In terms of R&D trends, there is a shift from single-target to multi-target, from injection to oral administration, and development of both peptides and small molecules.
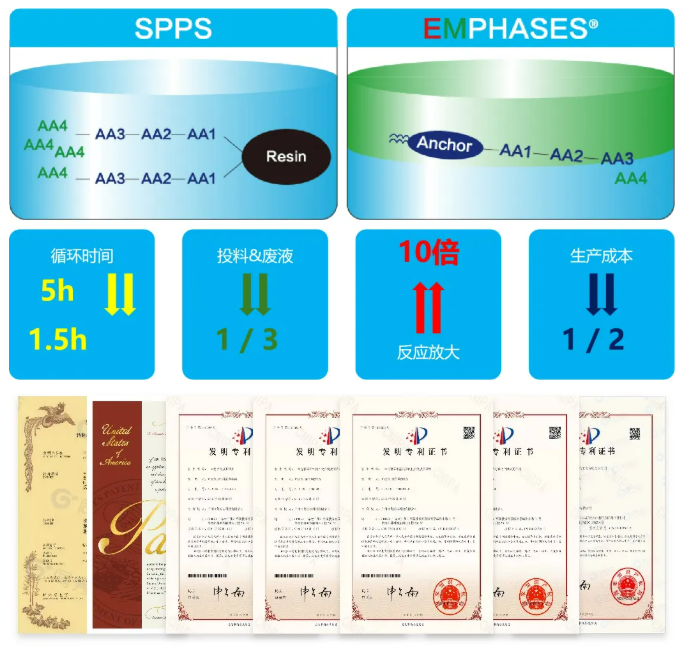
Congenpharma with EMPHASES ® Empowering the Industry with Peptide Synthesis Platform
Congen pharma focuses on the R&D and production of peptide and small molecule drugs, establishing the EMPHASES ® New liquid-phase peptide synthesis technology platform, using liquid-phase carriers to replace traditional solid-phase resins, improves reaction speed, shortens reaction time, and improves synthesis efficiency; reduces the use of amino acids, condensing agents, and solvents, reduces three-waste emissions, effectively controls costs, and has obtained 5 Chinese invention patents, 1 US patent, and 1 Japanese patent.
Key Business: ① Provide innovative process and quality research services for pharmaceutical peptides (including tirzepatide and semaglutide); ② Supply GLP-1 industrial chain products with quality and price advantages: side chains, short peptides, oral enhancers, etc.; ③ Supply advantageous fragments of Tirzepatide, Retatrutide, etc…...

CPHI China 2025, We cordially invite you to visit Congen's booth
From June 24th to 26th, the 23rd World Pharmaceutical Ingredients China Exhibition (CPHI China 2025) will be held at the Shanghai New International Expo Center. We cordially invite you to visit Congen pharma Booth E11D23 Exchange and discussion, join us in building a new blueprint for the GLP-1 industry!

Relevant Information
undefined
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
These cookies are necessary for basic functions such as payment. Standard cookies cannot be turned off and do not store any of your information.
These cookies collect information, such as how many people are using our site or which pages are popular, to help us improve the customer experience. Turning these cookies off will mean we can't collect information to improve your experience.
These cookies enable the website to provide enhanced functionality and personalization. They may be set by us or by third-party providers whose services we have added to our pages. If you do not allow these cookies, some or all of these services may not function properly.
These cookies help us understand what you are interested in so that we can show you relevant advertising on other websites. Turning these cookies off will mean we are unable to show you any personalized advertising.
Sorry,当前栏目暂无内容!
您可以查看其他栏目或返回 首页
Sorry,The current column has no content!
You can view other columns or return Home




