In-depth analysis of the permeation enhancement mechanism of Salcaprozate Sodium (SNAC): facilitating breakthroughs in oral delivery of macromolecular drugs
Publish Time:
2025-09-01
In the field of oral delivery of macromolecular drugs, effectively enhancing drug intestinal permeability and solving the problem of low oral bioavailability has always been a core focus of industry research. Among them, SNAC (N-8-(2-hydroxybenzamide) sodium octanoate), as an efficient permeation enhancer, provides a key solution for oral delivery of macromolecular drugs with its unique SNAC permeation mechanism, showing significant advantages especially in the field of peptide drugs.
Macromolecular drugs (such as peptide drugs) are mainly transported across intestinal epithelial cells via transcellular pathways through passive diffusion, active transport, or endocytosis, and increasing drug lipophilicity is key to improving intestinal transport efficiency. Among many methods to enhance peptide molecule hydrophobicity, SNAC (CAS No.: 203787-91-1) stands out due to its special interaction with drug molecules. As a small molecule carrier, SNAC can bind with peptide molecules through hydrophobic ion pairs, covalent or non-ionic interactions to form SNAC-peptide complexes, significantly increasing the lipophilicity of peptide molecules. The formation of hydrophobic ion pairs is similar to salt formation, where charged hydrophilic peptide molecules physically complex with oppositely charged SNAC (CAS No.: 203787-91-1). Leveraging the hydrophobic groups in SNAC, the water solubility of peptides is reduced and their lipophilicity enhanced, laying the foundation for drug penetration through the lipid bilayer membrane of intestinal epithelial cells.
From the core mechanism of SNAC (CAS No.: 203787-91-1) permeation enhancement, the hydrophobic aromatic group (2-hydroxybenzamide) on the SNAC molecule plays a key role. This group can interact hydrophobically with peptide molecules, altering their conformation to form SNAC-peptide complexes with higher lipophilicity. This conformational change and increased lipophilicity significantly enhance the complex's solubility in the cell lipid bilayer membrane, ultimately improving its transcellular passive transport efficiency. This non-covalent interaction is universal and has been validated in complexes formed between SNAC and various drugs such as heparin, insulin, and gliclazide. For example, in the ¹H NMR spectrum of the SNAC-gliclazide complex, the aromatic protons of SNAC show an upfield shift, directly proving the involvement of the 2-hydroxybenzamide group in interaction with gliclazide; for insulin, SNAC increases the lipophilic surface area by non-covalent bonding and conformational changes, exposing hydrophobic peptide regions that facilitate transcellular permeation, thereby enhancing insulin's transcellular permeability.
In specific drug application scenarios, the formation and mechanism of the semaglutide-SNAC complex are particularly typical. In non-enteric coated semaglutide tablets, SNAC rapidly binds with semaglutide in gastric juice to form a complex encapsulating semaglutide. This complex not only changes the local microenvironment pH around semaglutide (raising its pH), inhibits pepsin activity (which is more active in low pH), protecting semaglutide from degradation, but also significantly increases semaglutide permeability in the gastrointestinal tract through the SNAC permeation enhancement mechanism, providing core technical support for the clinical application of oral semaglutide formulations.
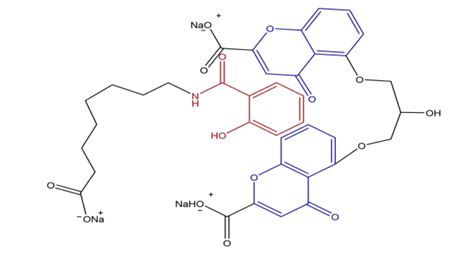
The hydrophobic aromatic group 2-hydroxybenzamide on the SNAC molecule
(in red) can insert into the two benzopyran rings of the gliclazide molecule due to hydrophobic interactions; (in blue) forming a complex with higher hydrophobicity
It is worth noting that the interaction between SNAC and gastrointestinal digestive fluids also affects its permeation enhancement effect, an important but often overlooked aspect of the SNAC permeation mechanism. Studies show that in the presence of 150 mmol/L SNAC, the permeability of drugs such as cyclosporine A, vancomycin, and ovalbumin in pig intestinal mucus changes. SNAC can enhance the viscoelasticity and physical barrier properties of pig intestinal mucus but has little effect on pig gastric mucus. This means that while SNAC can increase drug permeability in the gastrointestinal mucosa, it may also, by increasing mucus viscosity, somewhat affect drug molecule diffusion and absorption. This characteristic needs to be carefully considered in drug formulation development to achieve precise control of SNAC's permeation enhancement effect.
Besides the above pathways, SNAC's permeation enhancement performance in different drug systems further confirms the complexity and adaptability of its mechanism. For example, beyond peptide drugs, SNAC also modulates the intestinal permeability of some small and large molecule drugs (such as cyclosporine A) by forming a dynamic permeation balance through multiple interactions with drug molecules and the intestinal environment. With ongoing in-depth research into SNAC's permeation mechanism and precise control of its interactions with various drugs and intestinal environments, SNAC is expected to play a role in more oral delivery scenarios of macromolecular drugs, driving greater breakthroughs in the field of oral macromolecular drugs and providing patients with more convenient and efficient medication options.
Relevant Information
undefined





