Congenpharma GHK-Cu |Copper Tripeptide-1| manufacturer | Patented & Scale-Ready
Publish Time:
2025-09-19
Congenpharma Patented Process | Skin Remodeling & Anti-Cancer Defense
INCI: Copper Tripeptide-1
CAS: 49557-75-7
Purity: ≥99 % (HPLC) | Peptide content: ≥85 %
China IECIC-listed, vegan, cruelty-free, steroid-/hormone-free
CAS: 49557-75-7
Purity: ≥99 % (HPLC) | Peptide content: ≥85 %
China IECIC-listed, vegan, cruelty-free, steroid-/hormone-free
Patent & Awards
Manufactured under Congenpharma’s proprietary patent
“Method for synthesizing tripeptide-1 and copper peptide and its application” (ZL 202311370843.6)
2023 Guangdong Province “Famous & High-quality High-tech Product”
Manufactured under Congenpharma’s proprietary patent
“Method for synthesizing tripeptide-1 and copper peptide and its application” (ZL 202311370843.6)
2023 Guangdong Province “Famous & High-quality High-tech Product”
Green Scale-Up with EMPHASES®
Our new EMPHASIS™ continuous-flow platform cuts solvents by 62 %, reagents by 48 % and energy use by 35 % versus legacy batch methods.
Result: kilogram-scale GHK-Cu with a 29 % lower CO₂ footprint and 23 % lower cost—without touching purity or safety.
Our new EMPHASIS™ continuous-flow platform cuts solvents by 62 %, reagents by 48 % and energy use by 35 % versus legacy batch methods.
Result: kilogram-scale GHK-Cu with a 29 % lower CO₂ footprint and 23 % lower cost—without touching purity or safety.
Proven Performance
37 % firmer skin in 2 wk | 55 % wrinkle-depth reduction in 4 wk
32 % more collagen III in 8 wk | 68 % faster re-epithelialization
+34 % hair density after 12 wk | −42 % UV-B DNA damage (comet assay)
Anti-cancer: suppresses 70 % of genes over-expressed in metastatic epithelial cancers; non-mutagenic (Ames).Stimulates hair follicle anagen phase; +34 % hair-density after 12 weeks
37 % firmer skin in 2 wk | 55 % wrinkle-depth reduction in 4 wk
32 % more collagen III in 8 wk | 68 % faster re-epithelialization
+34 % hair density after 12 wk | −42 % UV-B DNA damage (comet assay)
Anti-cancer: suppresses 70 % of genes over-expressed in metastatic epithelial cancers; non-mutagenic (Ames).Stimulates hair follicle anagen phase; +34 % hair-density after 12 weeks
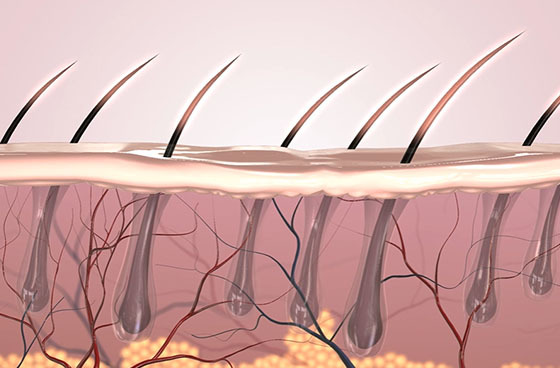
Formulation Friendly
Stable pH 4.5-7.5; compatible with retinoids, vitamin C, niacinamide, acids, peptides.
Add <40 °C; avoid >0.2 % EDTA.
Stable pH 4.5-7.5; compatible with retinoids, vitamin C, niacinamide, acids, peptides.
Add <40 °C; avoid >0.2 % EDTA.
Pack & Shelf
1 g, 10 g, 100 g pharma-alu pouch; 1 kg/20 bag-in-drum/box.
24 months at 2-8 °C.
“High purity ≥99% Copper Peptide (GHK-Cu)—remodel skin, defend genes, respect the planet.”
1 g, 10 g, 100 g pharma-alu pouch; 1 kg/20 bag-in-drum/box.
24 months at 2-8 °C.
“High purity ≥99% Copper Peptide (GHK-Cu)—remodel skin, defend genes, respect the planet.”
For samples, technical files or co-branding support: marketing@congenpharm.com
References
- 1.Pickart L. The human tri-peptide GHK and tissue remodeling. J. Biomater. Sci. Polym. Ed. 2008;19:969–988. doi: 10.1163/156856208784909435. [DOI] [PubMed] [Google Scholar]
- 2.Pickart L., Freedman J.H., Loker W.J., Peisach J., Perkins C.M., Stenkamp R.E., Weinstein B. Growth-modulating plasma tripeptide may function by facilitating copper uptake into cells. Nature. 1980;288:715–717. doi: 10.1038/288715a0. [DOI] [PubMed] [Google Scholar]
- 3.Lamb J. The Connectivity Map: A new tool for biomedical research. Nat. Rev. Cancer. 2007;7:54–60. doi: 10.1038/nrc2044. [DOI] [PubMed] [Google Scholar]
- 4.Pickart L., Vasquez-Soltero J.M., Margolina A. GHK and DNA: Resetting the human genome to health. BioMed Res. Int. 2014;2014:151479. doi: 10.1155/2014/151479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimoto E., Tanaka H., Gyotoku J., Morishige F., Pauling L. Enhancement of antitumor activity of ascorbate against Ehrlich ascites tumor cells by the copper: Glycylglycylhistidine complex. Cancer Res. 1983;43:824–828. [PubMed] [Google Scholar]
- 6.Pickart L. Ph.D. Thesis. University of California; San Francisco, CA, USA: 1973. A tripeptide in Human Serum That Promotes the Growth of Hepatoma Cells and the Survival of Normal Hepatocytes. [Google Scholar]
- 7.Maquart F.X., Pickart L., Laurent M., Gillery P., Monboisse J.C., Borel J.P. Stimulation of collagen synthesis in fibroblast cultures by the tripeptide-copper complex glycyl-l-histidyl-l-lysine-Cu2+ FEBS Lett. 1988;238:343–346. doi: 10.1016/0014-5793(88)80509-X. [DOI] [PubMed] [Google Scholar]
- 8.Siméon A., Wegrowski Y., Bontemps Y., Maquart F.X. Expression of glycosaminoglycans and small proteoglycans in wounds: Modulation by the tripeptide-copper complex glycyl-l-histidyl-l-lysine-Cu(2+) J. Investig. Dermatol. 2000;115:962–968. doi: 10.1046/j.1523-1747.2000.00166.x. [DOI] [PubMed] [Google Scholar]
- 9.Wegrowski Y., Maquart F.X., Borel J.P. Stimulation of sulfated glycosaminoglycan synthesis by the tripeptide-copper complex glycyl-l-histidyl-l-lysine-Cu2+ Life Sci. 1992;51:1049–1056. doi: 10.1016/0024-3205(92)90504-I. [DOI] [PubMed] [Google Scholar]
- 10.Siméon A., Monier F., Emonard H., Gillery P., Birembaut P., Hornebeck W., Maquart F.X. Expression and activation of matrix metalloproteinases in wounds: Modulation by the tripeptide-copper complex glycyl-l-histidyl-l-lysine-Cu2+ J. Investig. Dermatol. 1999;112:957–964. doi: 10.1046/j.1523-1747.1999.00606.x. [DOI] [PubMed] [Google Scholar]
- 11.Siméon A., Emonard H., Hornebeck W., Maquart F.X. The tripeptide-copper complex glycyl-l-histidyl-l-lysine-Cu2+ stimulates matrix metalloproteinase-2 expression by fibroblast cultures. Life Sci. 2000;22:57–65. doi: 10.1016/S0024-3205(00)00803-1. [DOI] [PubMed] [Google Scholar]
- 12.Huang P.J., Huang Y.C., Su M.F., Yang T.Y., Huang J.R., Jiang C.P. In vitro observations on the influence of copper peptide aids for the LED photoirradiation of fibroblast collagen synthesis. Photomed. Laser Surg. 2007;25:183–190. doi: 10.1089/pho.2007.2062. [DOI] [PubMed] [Google Scholar]
- 13.Kang Y.A., Choi H.R., Na J.I., Huh C.H., Kim M.J., Youn S.W., Kim K.H., Park K.C. Copper-GHK increases integrin expression and p63 positivity by keratinocytes. Arch. Dermatol. Res. 2009;301:301–306. doi: 10.1007/s00403-009-0942-x. [DOI] [PubMed] [Google Scholar]
- 14.Leyden J., Stephens T., Finkey M., Appa Y., Barkovic S. Skin Care Benefits of Copper Peptide Containing Facial Cream; Proceedings of the American Academy of Dermatology 60th Annual Meeting; New Orleans, LA, USA. 22–27 February 2002; p. 68. [Google Scholar]
- 15.Leyden J., Stephens T., Finkey M., Barkovic S. Skin Care Benefits of Copper Peptide Containing Eye Creams; Proceedings of the American Academy of Dermatology 60th Annual Meeting; New Orleans, LA, USA. 22–27 February 2002; p. 69. [Google Scholar]
- 16.Abdulghani A., Sherr A., Shirin S., Solodkina G., Tapia E., Wolf B., Gottlieb A.B. Effects of topical creams containing vitamin C, a copper-binding peptide cream and melatonin compared with tretinoin on the ultrastructure of normal skin—A pilot clinical, histologic, and ultrastructural study. Dis. Manag. Clin. Outcomes. 1998;1:136–141. doi: 10.1016/S1088-3371(98)00011-4. [DOI] [Google Scholar]
- 17.Finkley M., Appa Y., Bhandarkar S. Copper Peptide and Skin. In: Elsner P., Maibach H., editors. Cosmeceuticals and Active Cosmetics: Drugs vs. Cosmetics. Marcel Dekker; New York, NY, USA: 2005. pp. 549–563. [Google Scholar]
- 18.Krüger N., et al. Topische Applikation eines Kupfertripeptidkomplexes: Pilotstudie bei gealterter Haut. J. Dtsch. Dermatol. Ges. 2003;1 [Google Scholar]
- 19.Krüger N., Fiegert L., Becker D., Reuther T., Kerscher M. Zur Behandlung der Hautalterung: Spurenelemente in Form eines Kupfertripeptidkomplexes. Kos. Med. 2003;24:31–33. [Google Scholar]
- 20.Badenhorst T., Svirskis D., Merrilees M., Bolke L., Wu Z. Effects of GHK-Cu on MMP and TIMP Expression, Collagen and Elastin Production, and Facial Wrinkle Parameters. J. Aging Sci. 2016;4:166. doi: 10.4172/2329-8847.1000166. [DOI] [Google Scholar]
- 21.Cangul I.T., Gul N.Y., Topal A., Yilmaz R. Evaluation of the effects of topical tripeptide-copper complex and zinc oxide on open-wound healing in rabbits. Vet. Dermatol. 2006;17:417–423. doi: 10.1111/j.1365-3164.2006.00551.x. [DOI] [PubMed] [Google Scholar]
- 22.Gul N.Y., Topal A., Cangul I.T., Yanik K. The effects of topical tripeptide copper complex and helium-neon laser on wound healing in rabbits. Vet. Dermatol. 2008;19:7–14. doi: 10.1111/j.1365-3164.2007.00647.x. [DOI] [PubMed] [Google Scholar]
- 23.Arul V., Gopinath D., Gomathi K., Jayakumar R. Biotinylated GHK peptide incorporated collagenous matrix: A novel biomaterial for dermal wound healing in rats. J. Biomed. Mater. Res. B Appl. Biomater. 2005;73:383–391. doi: 10.1002/jbm.b.30246. [DOI] [PubMed] [Google Scholar]
- 24.Arul V., Kartha R., Jayakumar R. A therapeutic approach for diabetic wound healing using biotinylated GHK incorporated collagen matrices. Life Sci. 2007;80:275–284. doi: 10.1016/j.lfs.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Canapp S.O., Jr., Farese J.P., Schultz G.S., Gowda S., Ishak A.M., Swaim S.F., Vangilder J., Lee-Ambrose L., Martin F.G. The effect of topical tripeptide-copper complex on healing of ischemic open wounds. Vet. Surg. 2003;32:515–523. doi: 10.1111/j.1532-950X.2003.00515.x. [DOI] [PubMed] [Google Scholar]
- 26.Lane T.F., Iruela-Arispe M.L., Johnson R.S., Sage E.H. SPARC is a source of copper-binding peptides that stimulate angiogenesis. J. Cell Biol. 1994;125:929–943. doi: 10.1083/jcb.125.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sensenbrenner M., Jaros G.G., Moonen G., Meyer B.J. Effect of conditioned media on nerve cell differentiation. Cell. Mol. Life Sci. 1980;36:660–662. doi: 10.1007/BF01970123. [DOI] [PubMed] [Google Scholar]
- 28.Lindner G., Grosse G., Halle W., Henklein P. The effect of a synthetic tripeptide nervous tissue cultured in vitro. Z. Mikrosk. Anat. Forsch. 1979;93:820–828. [PubMed] [Google Scholar]
- 29.Ahmed M.R., Basha S.H., Gopinath D., Muthusamy J., Jayakumar R.J. Initial upregulation of growth factors and inflammatory mediators during nerve regeneration in the presence of cell adhesive peptide-incorporated collagen tubes. J. Peripher. Nerv. Syst. 2005;10:17–30. doi: 10.1111/j.1085-9489.2005.10105.x. [DOI] [PubMed] [Google Scholar]
- 30.Pickart L., Vasquez-Soltero J., Margolina A. The Effect of the Human Peptide GHK on Gene Expression Relevant to Nervous System Function and Cognitive Decline. Brain Sci. 2017;7:20. doi: 10.3390/brainsci7020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cebrian J., Messeguer A., Facino R., Garcia Anton J. New anti-RNS and -RCS products for cosmetic treatment. Int. J. Cosmet. Sci. 2005;27:271–278. doi: 10.1111/j.1467-2494.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- 32.Thomas C.E. The influence of medium components on Cu(2+)-dependent oxidation of low-density lipoproteins and its sensitivity to superoxide dismutase. Biochim. Biophys. Acta. 1992;1128:50–57. doi: 10.1016/0005-2760(92)90256-U. [DOI] [PubMed] [Google Scholar]
- 33.Beretta G., Arlandini E., Artali R., Anton J.M., Maffei Facino R. Acrolein sequestering ability of the endogenous tripeptide glycyl-histidyl-lysine (GHK): Characterization of conjugation products by ESI-MSn and theoretical calculations. J. Pharm. Biomed. Anal. 2008;47:596–602. doi: 10.1016/j.jpba.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Beretta G., Artali R., Regazzoni L., Panigati M., Facino R.M. Glycyl-histidyl-lysine (GHK) is a quencher of alpha,beta-4-hydroxy-trans-2-nonenal: A comparison with carnosine. insights into the mechanism of reaction by electrospray ionization mass spectrometry, 1H NMR, and computational techniques. Chem. Res. Toxicol. 2007;20:1309–1314. doi: 10.1021/tx700185s. [DOI] [PubMed] [Google Scholar]
- 35.Miller D.M., DeSilva D., Pickart L., Aust S.D. Effects of glycyl-histidyl-lysyl chelated Cu(II) on ferritin dependent lipid peroxidation. Adv. Exp. Med. Biol. 1990;264:79–84. doi: 10.1007/978-1-4684-5730-8_11. [DOI] [PubMed] [Google Scholar]
- 36.Boo S., Dagnino L. Integrins as Modulators of Transforming Growth Factor Beta Signaling in Dermal Fibroblasts During Skin Regeneration After Injury. Adv. Wound Care. 2013;2:238–246. doi: 10.1089/wound.2012.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell J.D., McDonough J.E., Zeskind J.E., Hackett T.L., Pechkovsky D.V., Brandsma C.A., Suzuki M., Gosselink J.V., Liu G., Alekseyev Y.O., et al. A gene expression signature of emphysema-related lung destruction and its reversal by the tripeptide GHK. Genome Med. 2012;4:67. doi: 10.1186/gm367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meiners S., Eickelberg O. Next-generation personalized drug discovery: The tripeptide GHK hits center stage in chronic obstructive pulmonary disease. Genome Med. 2012;4:70. doi: 10.1186/gm371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park J.R., Lee H., Kim S., Yang S.R. The tri-peptide GHK-Cu complex ameliorates lipopolysaccharide-induced acute lung injury in mice. Oncotarget. 2016;7:58405. doi: 10.18632/oncotarget.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coondoo A., Phiske M., Verma S., Lahiri K. Side-effects of topical steroids: A long overdue revisit. Indian Dermatol. Online J. 2014;5:416–425. doi: 10.4103/2229-5178.142483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pickart L. Method of Using Copper(II) Containing Compounds to Accelerate Wound Healing. 5,164,367. U.S. Patent. 1992 Nov 17;
- 42.Heinrich J., Balleisen L., Schulte H., Assmann G., van de Loo J. Fibrinogen and factor VII in the prediction of coronary risk. Results from the PROCAM study in healthy men. Arterioscler. Thromb. Vasc. Biol. 1994;14:54–59. doi: 10.1161/01.ATV.14.1.54. [DOI] [PubMed] [Google Scholar]
- 43.Lee A.J., Smith W.C.S., Lowe G.D.O., Tunstall-Pedoe H. Plasma fibrinogen and coronary risk factors: The Scottish Heart Health Study. J. Clin. Epidemiol. 1990;43:913–919. doi: 10.1016/0895-4356(90)90075-Z. [DOI] [PubMed] [Google Scholar]
- 44.Pickart L., Vasquez-Soltero J.M., Margolina A. Resetting Skin Genome Back to Health Naturally with GHK. In: Farage M.A., Miller K.W., Maibach H.I., editors. Textbook of Aging Skin. Springer; Berlin, Germany: 2017. [DOI] [Google Scholar]
- 45.Hong Y., Downey T., Eu K., Koh P., Cheah P. A “metastasis-prone” signature for early-stage mismatch-repair proficient sporadic colorectal cancer patients and its implications for possible therapeutics. Clin. Exp. Metastasis. 2010;27:83–90. doi: 10.1007/s10585-010-9305-4. [DOI] [PubMed] [Google Scholar]
- 46.Pickart L., Vasquez-Soltero J.M., Margolina A. GHK Peptide as a Natural Modulator of Multiple Cellular Pathways in Skin Regeneration. BioMed Res. Int. 2015;2015:648108. doi: 10.1155/2015/648108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rugină D., Hanganu D., Diaconeasa Z., Tăbăran F., Coman C., Leopold L., Bunea A., Pintea A. Antiproliferative and Apoptotic Potential of Cyanidin-Based Anthocyanins on Melanoma Cells. Int. J. Mol. Sci. 2017;18:949. doi: 10.3390/ijms18050949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matalka L.E., Ford A., Unlap M.T. The tripeptide, GHK, induces programmed cell death in SH-SY5Y neuroblastoma cells. J. Biotechnol. Biomater. 2012;2:1–4. doi: 10.4172/2155-952X.1000144. [DOI] [Google Scholar]
- 49.Pickart L., Vasquez-Soltero J.M., Pickart F.D., Majnarich J. GHK, the Human Skin Remodeling Peptide, Induces Anti-Cancer Expression of Numerous Caspase, Growth Regulatory, and DNA Repair Genes. Anal. Oncol. 2014;3:79–89. doi: 10.6000/1927-7229.2014.03.02.2. [DOI] [Google Scholar]
- 50.Imbert I., Gondran C., Oberto G., Cucumel K., Dal Farra C., Domloge N. Maintenance of the ubiquitin proteasome system activity correlates with visible skin benefits. Int. J. Cosmet. Sci. 2010;32:446–457. doi: 10.1111/j.1468-2494.2010.00575.x. [DOI] [PubMed] [Google Scholar]
- 51.Ventafridda V., Fochi C., De Conno D., Sganzerla E. Use of non-steroidal anti-inflammatory drugs in the treatment of pain in cancer. Br. J. Clin. Pharmacol. 1980;10(Suppl. 2):343S–346S. doi: 10.1111/j.1365-2125.1980.tb01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young M.D., Lottes S.R., Webb L.A. An evaluation of cimetidine and ranitidine in the pain relief and acute healing of duodenal ulcer disease. Clin. Ther. 1988;10:543–552. [PubMed] [Google Scholar]
- 53.Bobyntsev I.I., Chernysheva O.I., Dolgintsev M.E., Smakhtin M.Y., Belykh A.E. Anxiolytic Effects of Gly-His-Lys Peptide and Its Analogs. Bull. Exp. Biol. Med. 2015;156:726–728. doi: 10.1007/s10517-015-2847-3. [DOI] [PubMed] [Google Scholar]
- 54.Chernysheva O.I., Bobyntsev I.I., Dolgintsev M.E. The tripeptide GLY-HIS-LYS influence on behavior of rats in the test “open field”. Adv. Mod. Biol. Sci. 2014;12:357–360. [Google Scholar]
- 55.Sever’yanova L.А., Dolgintsev M.E. Effects of Tripeptide Gly-His-Lys in Pain-Induced Aggressive-Defensive Behavior in Ra. Bull. Exp. Biol. Med. 2017;164:140–143. doi: 10.1007/s10517-017-3943-3. [DOI] [PubMed] [Google Scholar]
- 56.Hostynek J.J., Dreher F., Maibach H.I. Human skin retention and penetration of a copper tripeptide in vitro as function of skin layer towards anti-inflammatory therapy. Inflamm. Res. 2010;59:983–988. doi: 10.1007/s00011-010-0214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hall J.M., Cruser D., Podawiltz A., Mummert D.I., Jones H., Mummert M.E. Psychological Stress and the Cutaneous Immune Response: Roles of the HPA Axis and the Sympathetic Nervous System in Atopic Dermatitis and Psoriasis. Dermatol. Res. Pract. 2012;2012:403908. doi: 10.1155/2012/403908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwartz J., Evers A.W., Bundy C., Kimball A.B. Getting under the Skin: Report from the International Psoriasis Council Workshop on the Role of Stress in Psoriasis. Front. Psychol. 2016;7:87. doi: 10.3389/fpsyg.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson H., Norton S., Jarrett P., Broadbent E. The effects of psychological interventions on wound healing: A systematic review of randomized trials. Br. J. Health Psychol. 2017;22:805–835. doi: 10.1111/bjhp.12257. [DOI] [PubMed] [Google Scholar]
- 60.Brown J. The impact of stress on acute wound healing. Br. J. Community Nurs. 2016;21(Suppl. 12):S16–S22. doi: 10.12968/bjcn.2016.21.Sup12.S16. [DOI] [PubMed] [Google Scholar]
- 61.Mazurowska L., Mojskin M. Biological activities of selected peptides: Skin penetration ability of copper complexes with peptides. J. Cosmet. Sci. 2008;59:59–69. [PubMed] [Google Scholar]
- 62.Mazurowska L., Mojski M. ESI-MS study of the mechanism of glycyl-l-histidyl-l-lysine-Cu(II) complex transport through model membrane of stratum corneum. Talanta. 2007;72:650–654. doi: 10.1016/j.talanta.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 63.Swaminathan J., Ehrhardt C. Liposomal delivery of proteins and peptides. Expert Opin. Drug Deliv. 2012;9:1489–1503. doi: 10.1517/17425247.2012.735658. [DOI] [PubMed] [Google Scholar]
- 64.Lalioti V., Muruais G., Tsuchiya Y., Pulido D., Sandoval I.V. Molecular mechanisms of copper homeostasis. Front. Biosci. 2009;14:4878–4903. doi: 10.2741/3575. [DOI] [PubMed] [Google Scholar]
- 65.Szarcvel Szic K., Declerck K., Vidaković M., Vanden B.W. From inflammaging to healthy aging by dietary lifestyle choices: Is epigenetics the key to personalized nutrition? Clin. Epigenet. 2015;7:33. doi: 10.1186/s13148-015-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaliman P., Alvarez-López M.J., Cosín-Tomás M., Rosenkranz M.A., Lutz A., Davidson R.J. Rapid changes in histone deaconesses and inflammatory gene expression in expert meditators. Psychoneuroendocrinology. 2014;40:96–107. doi: 10.1016/j.psyneuen.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prev:
Relevant Information
undefined





