-
-
GLP-1 Side Chains
 显示更多
显示更多 -
Peptide Intermediates

-
Fmoc-His-Aib-OH
-
Fmoc-Ile-Aib-OH
-
Fmoc-Tyr(tBu)-Aib-OH
-
Fmoc-Glu(OtBu)-Aib-OH
-
Fmoc-His(Fmoc)-Aib-OH
-
Fmoc-His(Fmoc)-Aib-OSu
显示更多 -
-
Tirzepatide Fragments

-
CAS:2682040-93-1
-
CAS:3034670-52-2
-
CAS:2656383-23-0
-
CAS:2461524-68-3
-
CAS:2656383-24-1
-
CAS:2656383-25-2
显示更多 -
-
API & Excipients
 显示更多
显示更多 -
Cosmetic Peptides
-
Oligopeptide-1
-
NonaPeptide-1
-
Copper Tripeptide-1
-
Acetyl Hexapeptide-8
-
Palmitoyl Tripeptide-1
-
Decarboxy Carnosine HCl
显示更多 -
-
Active Ingredients
 显示更多
显示更多
-
Structure Analysis of Tirzepatide: Pharmacodynamic Mechanism and Key Intermediates
Publish Time:
2025-08-22
Tirzepatide, as a dual agonist of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors, has become an important drug in the field of metabolic disease treatment due to its synergistic metabolic regulation and convenient once-weekly subcutaneous injection administration. High-quality intermediates and efficient peptide synthesis technology are the core supports to ensure the production of this drug.

The function of Tirzepatide
The structure and pharmacodynamics of Tirzepatide
Tirzepatide is a linear peptide containing 39 amino acids, optimized based on the natural GIP structure, similar in size to GIP and GLP-1. The initial amino acid sequence of Tirzepatide is the human GIP sequence, retaining 9 homologous amino acids from GIP and 10 amino acids common to both GIP and GLP-1, achieving dual receptor agonism and long-acting effects through multi-site structural modifications.
C20 contains a fatty diacid structure, connected to a lysine residue via a linker, promoting binding to albumin and extending the half-life. Position 2 contains an α-amino isobutyric acid (Aib) residue, which occupies the dipeptidyl peptidase-4 (DPP-4) binding site, preventing hydrolysis by DPP-4. The 10 amino-terminal amino acids are the same as those in the exenatide amino acid series. *

The structure of Tirzepatide
Tirzepatide intermediates
In the preparation process of Tirzepatide, the synthesis of intermediates, side chains, and fragments is crucial and is the key to ensuring efficient and stable drug production.
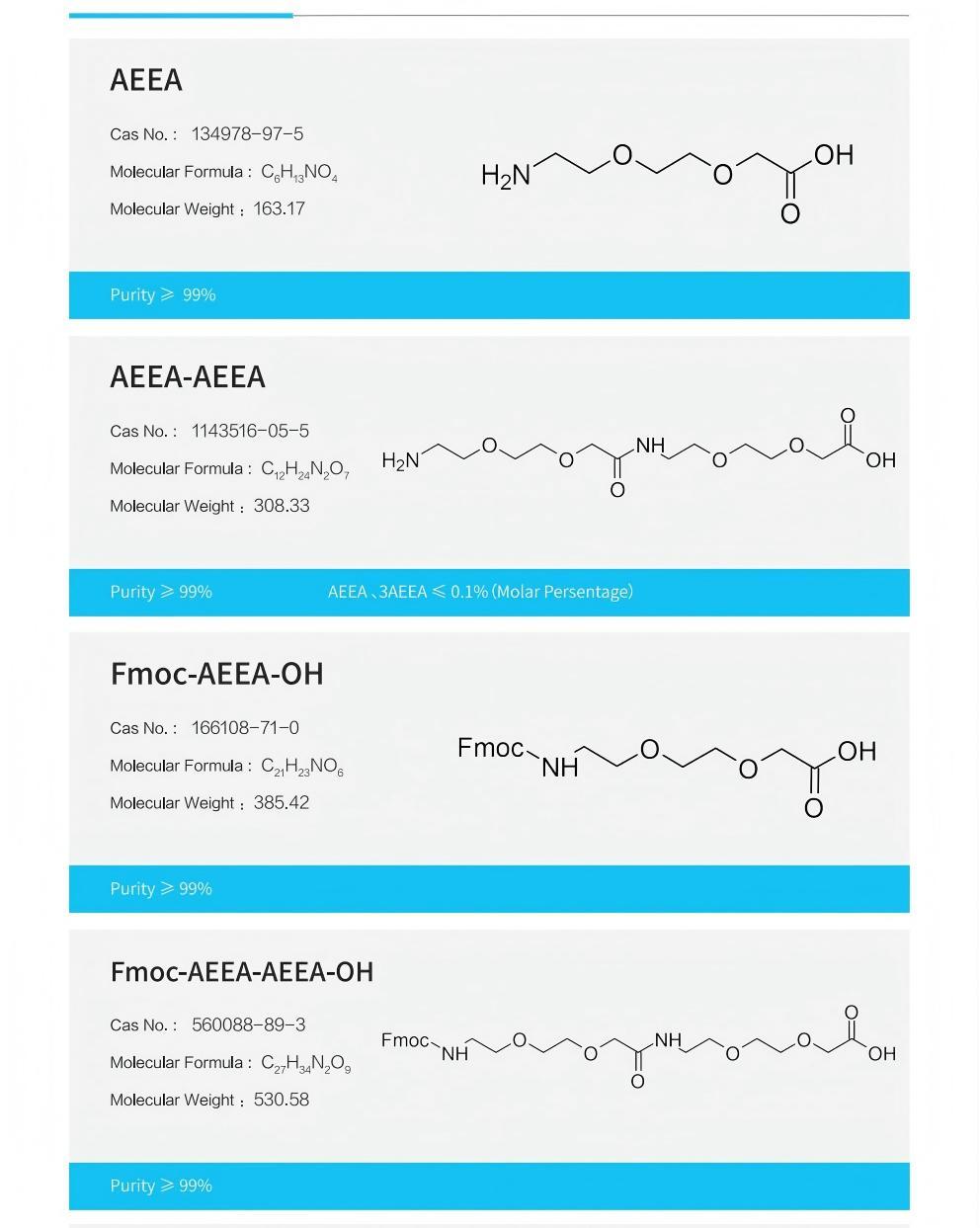
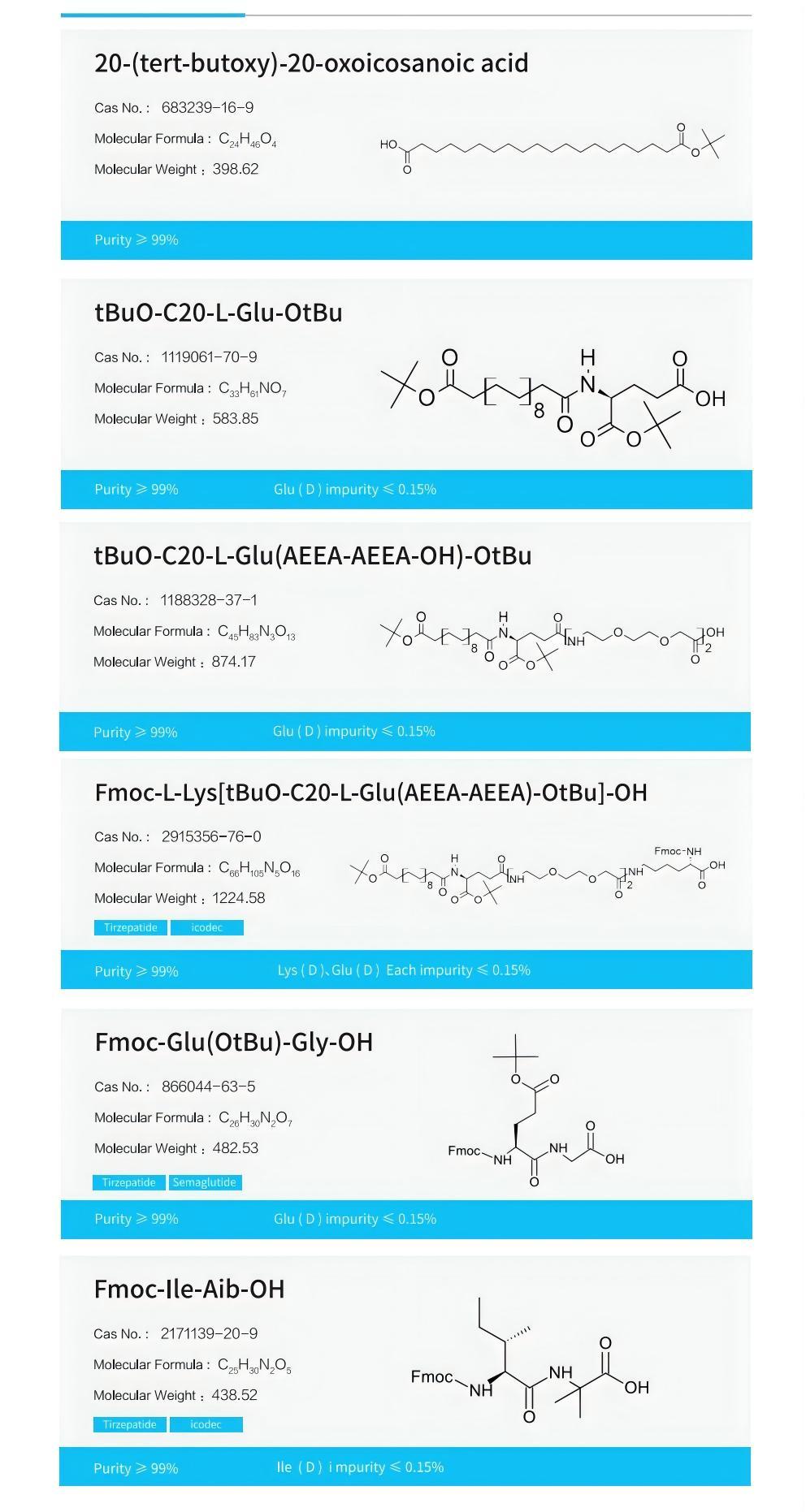
Tongjun Pharmaceuticals is committed to providing sustainable and affordable peptides, supplying side chains Key hydrophilic linkers in the AEEA series intermediates: AEEA, AEEA-AEEA, Fmoc-AEEA-OH, produced using a self-developed process different from existing technologies, free of β-alanine impurities, with high purity and stable quality;
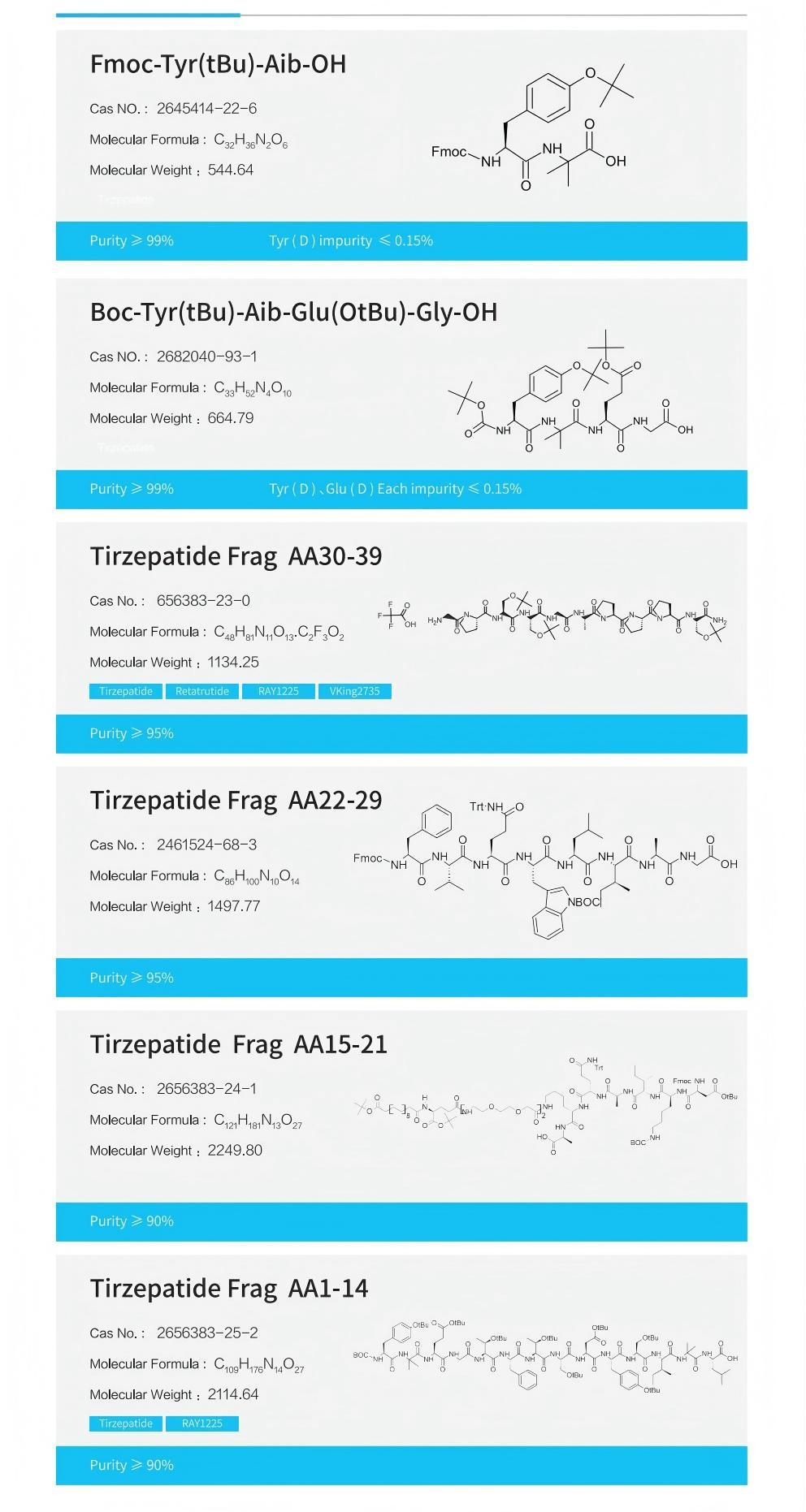
Using EMPHASES ® Peptide fragments (≥8 amino acids) produced by the new liquid-phase peptide synthesis platform, with purity greater than 95%;
The four fragments of the original process have completed small-scale and pilot-scale verification, and fragments different from the original process can be customized as required, suitable for Tirzepatide process development and optimization.
[Related Directory]
*Zhang Yanping, Huang Bin, Lu Jinmiao, et al. The first GIP/GLP-1 dual receptor agonist: Tirzepatide [J]. Chinese Journal of Clinical Pharmacy, 2025, 34(07):533-539. DOI:10.19577/j.1007-4406.2025.07.011.
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
These cookies are necessary for basic functions such as payment. Standard cookies cannot be turned off and do not store any of your information.
These cookies collect information, such as how many people are using our site or which pages are popular, to help us improve the customer experience. Turning these cookies off will mean we can't collect information to improve your experience.
These cookies enable the website to provide enhanced functionality and personalization. They may be set by us or by third-party providers whose services we have added to our pages. If you do not allow these cookies, some or all of these services may not function properly.
These cookies help us understand what you are interested in so that we can show you relevant advertising on other websites. Turning these cookies off will mean we are unable to show you any personalized advertising.
Sorry,当前栏目暂无内容!
您可以查看其他栏目或返回 首页
Sorry,The current column has no content!
You can view other columns or return Home




